Patent name: A N, P/RC @ Pb as carbon source of lead battery cathode carbon additives, the preparation methods of negative plate and negative plate

The inventor: Shao Yonggang, Xie Fa, shao-hua Yang, Zhang Meng, sides lighted, Zhang Daode
The irreversible sulfation of the negative electrode of lead-acid batteries is the main cause of battery failure at HRPSoC and low temperature. By adding carbon materials to the negative active material to make a "lead carbon battery", the battery performance has been greatly improved, and the commonly used additives are activated carbon, graphite, carbon nanotubes and graphene. Because the hydrogen evolution potential of the carbon material itself is relatively low, after the battery reaches the end of charging, a very serious hydrogen evolution phenomenon will occur in the carbon material, causing the battery to lose water seriously, reducing the charging efficiency of the battery, and greatly affecting the performance of the battery.
This patent presents a with N and P/RC @ Pb as carbon source of lead battery cathode carbon additives. The hydrogen evolution reaction can be inhibited while the sulfation of the negative electrode is reduced. Using macroporylaminophosphonic acid chelating resin as carbon source, Pb ion was adsorbed to achieve close combination of Pb and C to obtain N, P/RC@Pb composite . As an additive, it is directly added to the lead paste semi-finished product (adding amount 0.5~2%) to prepare the negative plate. macroporous amino phosphonic acid base chelating resin is a kind of rough surface and internal hollow spherical organic polymer. Rich in nitrogen and phosphorus, it has the characteristics of large specific surface area, good adsorption performance, low price and simple preparation process. Calcined at high temperature, the carbon material obtained has the characteristics of high nitrogen and high phosphorus. The effective reaction area between electrolyte and electrode plate can be effectively improved and the electrochemical performance of the battery can be improved when used in the negative electrode material of lead-carbon battery. Therefore, the N, P/RC@Pb composite material of the invention contains rich nitrogen and phosphorus elements and carbon elements, and has the advantages of large specific surface area, stable chemical properties, environmental protection and the like. It is a kind of spherical composite material with a large hole embedded with Pb substance on the surface and a hollow inside. The adsorbed Pb ion reacts with P, O and other elements in the macroporous aminophosphinic acid chelating resin during calcination to obtain Pb oxide, thus achieving a close connection between Pb and C material. N, P/RC @ the preparation technology of Pb composites: to macroporous amino phosphonic acid base chelating resin blend to shock and lead solution, take out the samples, after washing dry, get precursor; The precursor is heated and kept warm in an inert atmosphere, and then ground after cooling to obtain N, P/RC@Pb composite material. lead carbon batteries of the present invention to negative plate preparation: to N, P/RC @ Pb composites and lead paste semi-finished blend to add water, stirring after get mixture; The mixture is coated on the negative plate grid, pressed flat, doused with acid, cured, dried, and obtained.
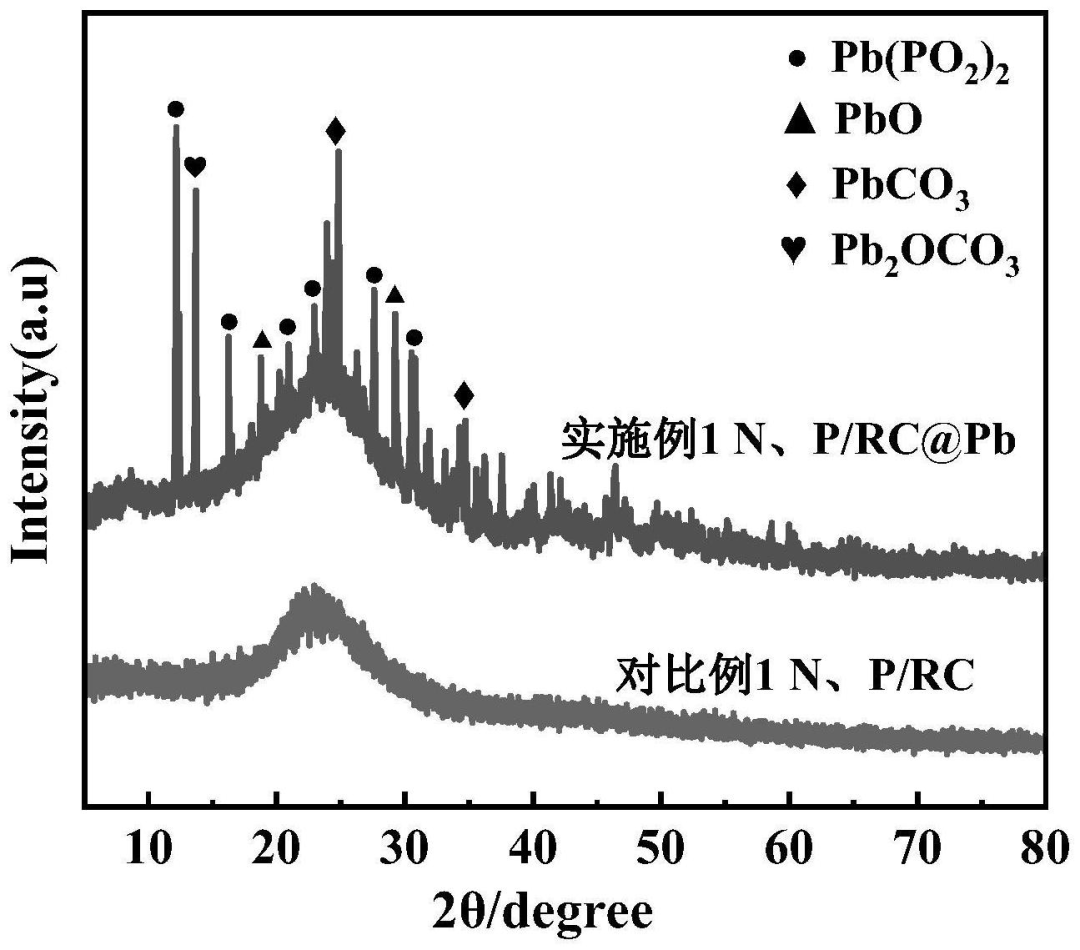
Preparation of N, P/RC @ Pb composite materials and N, P/RC XRD test the result is shown in figure 1: N and P/RC show two wide diffraction peaks at 22.5° and 43°, which are related to amorphous carbon, indicating the presence of carbon in the material. The peaks at 2θ = 26° and 44° belong to the (002) and (101) diffraction of the graphite skeleton, indicating that the heat treatment promotes the formation of some graphite structures. N, P/RC@Pb composites not only have the corresponding N and P/RC diffraction peaks, but also have the oxide peaks of Pb.The prepared N, P/RC@Pb composite material was tested by SEM (Figure 2). The picture on the right is a partial enlargement of the marked part of the frame on the left. It can be seen that the carbonized material still has a hollow spherical structure, which is due to the macroporous amino phosphonic acid chelate resin has such a structure. 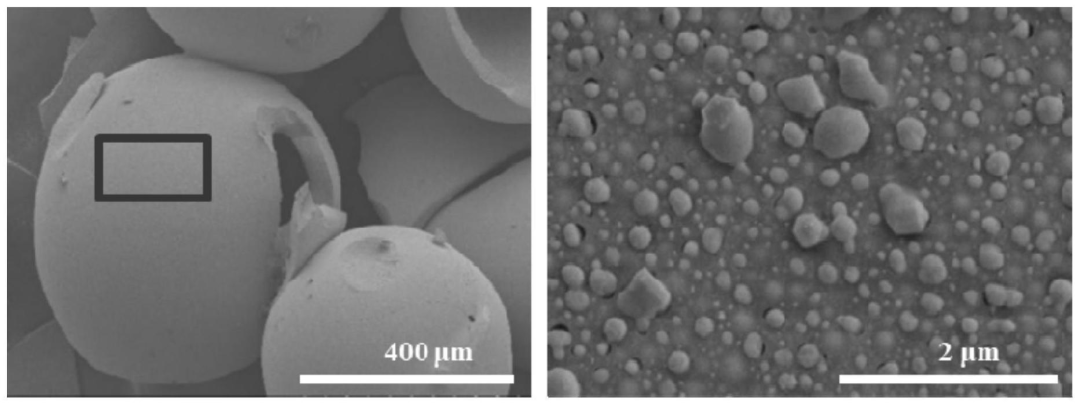
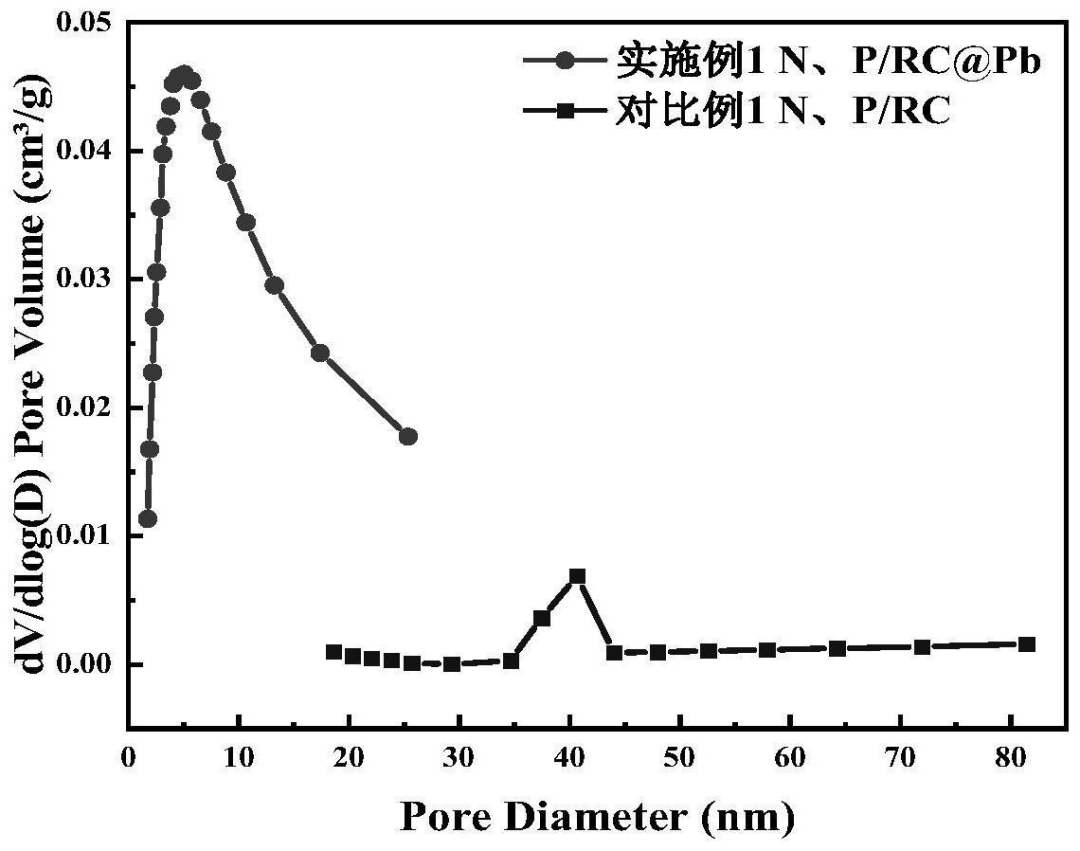
The BET test results of are shown in FIG. 3 (adsorption desorption curve) and FIG. 4 (pore size distribution diagram). The adsorption desorption curves of N, P/RC and N, P/RC@Pb are typical type III isotherms, belonging to the material with large pores. The pore size distribution peaks of N and P/RC range from 30 nm to 50nm, indicating that the material forms medium and large pores. The pore size distribution peaks of N and P/RC@Pb range from 0 to 15nm, indicating that the pore size of N and P/RC will be reduced and the pore size of N and P/RC@Pb will be increased when Pb ions are absorbed and carbonized. This conforms to the corresponding N 2 stripping absorption curve. N, P/RC specific surface area and pore volume of 9.58 m / 2 g and 0. 7 cm / 3 g, The specific surface area and pore volume of N and P/RC@Pb are 22.19m2/g and 0.04cm3/g. By comparison, it is found that the specific surface area of N and P/RC@Pb increases greatly and the pore volume decreases, indicating that the pore size of large pores decreases and the number of micro and medium pores increases. Causing its specific surface area to increase. 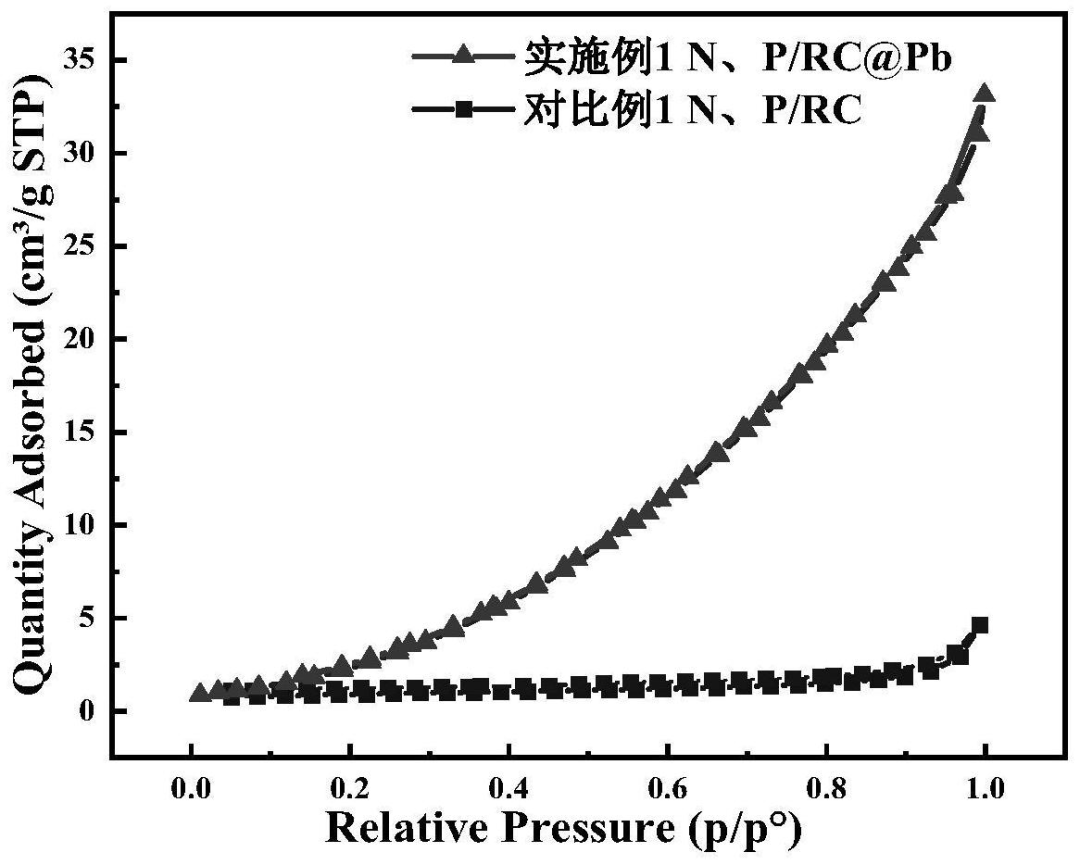
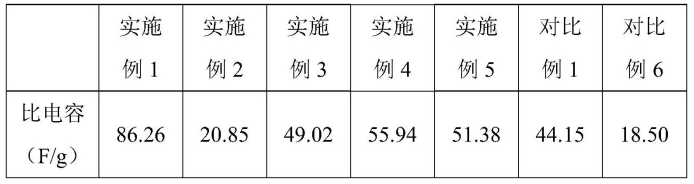
Adopting a three-electrode system (embodiments or pairs of proportional working electrodes, Hg/Hg2 SO4 is the reference electrode, and Pt is the opposite electrode.) Electrochemical tests are performed on the negative plate, and the calculation results of specific capacitance (see Table 1) show that, Embodiment 1 (1% N, P/RC@Pb) has the largest capacity compared with proportion 1 (1% N, P/RC) and proportion 6 (blank group). The main reason is that the doping of Pb can inhibit the hydrogen evolution reaction caused by carbon materials (N, P/RC materials) to a certain extent, and alleviate the water loss problem of the battery; In addition, PB-doped carbon materials (N, P/RC@Pb materials) can increase the material density, reduce the agglomeration problem caused by carbon materials (N, P/RC materials) due to low density and light weight, and further improve the beneficial role of carbon in the electrode plate.
Table 1. Negative contrast specific capacitance
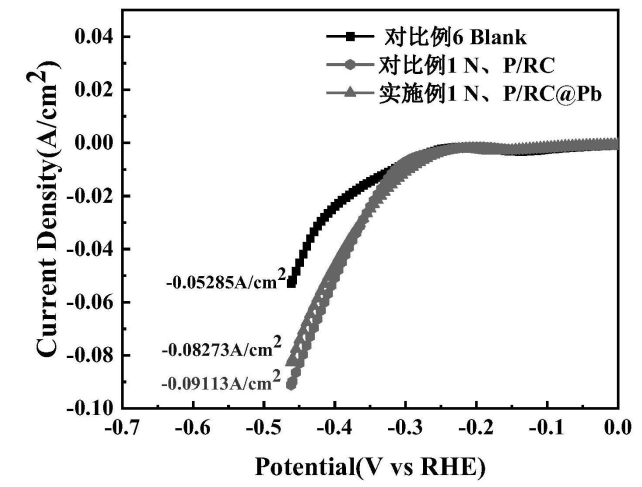
Measured by three electrodes system {{LSV diagram (FIG. 14), under the same hydrogen evolution voltage, the hydrogen evolution current densities of 1% N, P/RC@Pb (Embodiment 1), 1% N, P/RC (for ratio 1) and Blank group (for ratio 6 Blank) were added as follows: blank < 1% N, P/RC@Pb < 1% N, P/RC.
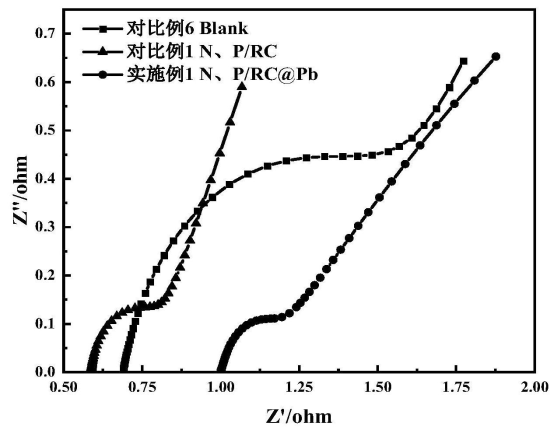
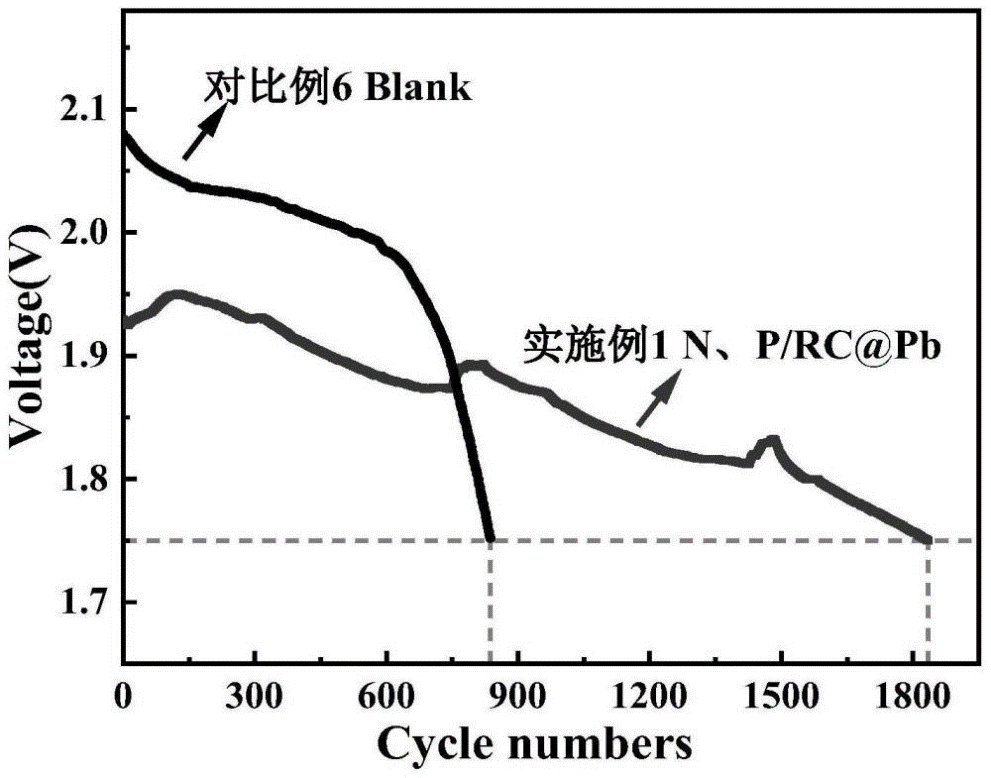
The measured EIS eIS by a three-electrode system is shown in FIG. 6, where the resistance of 1% N and P/RC@Pb is the lowest, the semi-arc of the high frequency region is the lowest, and the slope of the low frequency region is the highest, which is due to the addition of N and P/RC@Pb composite materials. The negative plate has greater electrochemical activity and active surface area. It is prepared into a closed analog battery, and the cycle life of battery (2C60s charge and discharge cycle) is tested after formation. The results show (FIG. 7) that the cycle numbers of blank battery, 1% N and P/RC@Pb under HRPSoC operation are 867 and 1148, respectively, which proves that the addition of N and P/RC@Pb composites significantly increases the cycle life of the battery.